温馨提示:手机用户请点击下方“原网页”或“电脑版”进行查看本文,效果最佳!
一起学习吧高考网为你带来厦门市2016-2017学年第一学期期末质量检测高一化学试题答案,请大家多多关注本站为你提供的第一手考试资讯。
一、选择题(每小题仅有1个正确答案,各3分,共45分)
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
|
A |
B |
C |
B |
B |
A |
B |
C |
C |
B |
A |
C |
D |
A |
D |
二、填空题(共55分)
16.(10分)
(1)2NH
4Cl + Ca(OH)
2 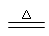
2NH
3↑+ CaCl
2+ 2H
2O (2分)
(2)2NaBr + Cl
2 = 2NaCl + Br
2 (2分)
(3)C + 2H
2SO
4(浓)

CO
2↑+ 2SO
2↑+ 2H
2O (2分)
(4)2Fe
2++Cl
2 = 2Fe
3++2Cl
- (2分)
(5)3Cu + 2NO
3-+ 8H
+ =3Cu
2++ 2NO↑+ 4H
2O (2分)
17.(10分)
(1)C (1分)
(2)11.2 (1分)
NA (或6.02×10
23) (1分)
30 (1分)
(3)250 mL容量瓶 (1分)
25.0 (1分)
AC (各1分,共2分)
(4)4NH
3+6NO 催化剂 5N
2+6H
2O (2分)
18.(11分)
(1)①B (1分)
硫酸浓度太小,SO
2会大量溶于水中难以逸出 (2分)
②e、f、g、h、i (2分)
(2)①淡黄 (1分)
②用带火星的木条靠近Z装置的气体出口 (1分)
③木条复燃 (1分)
④溴水褪色 (1分)
⑤取步骤III中滤液,先加入足量稀盐酸,再加入BaCl
2溶液,如果得到白色沉淀,说明Y中固体中含有Na
2SO
4 (2分)
19.(11分)
(1)CaO (1分)
(2)O
2 (1分)
0.004
NA (1分)
(3)①H
2 (1分)
② 2Fe
3+ + Fe = 3Fe
2+ (2分)
2H
+ + Fe = H
2↑ + Fe
2+ (2分)
< (1分)
③滴加KSCN溶液,无明显现象 (2分)
20.(13分)
(1)+5 (1分)
(2)KCl、Cl
2 (各1分,共2分)
(3)复分解反应(或中和反应) (1分)
(4)AC (各1分,共2分)
(5)蒸发浓缩、冷却结晶 (1分)
(2)①蓝色溶液变无色 (1分)
② KIO
3 ~ 3 I
2 ~ 6 Na
2S
2O
3
1 6
n (KIO
3) 2.0×12.00×10
-3mol (1分)
n (KIO
3) = 4×10
-3mol (1分)
ω(KIO
3) = [(4×10
-3mol×214g/mol) / 1.0g]×100% =85.6% (1分)
③ 4I
― + O
2 + 4H
+ = 2I
2 + 2H
2O (2分)
高中化学模拟题 http://www.17xuexiba.com/hx/
 2NH3↑+ CaCl2+ 2H2O (2分)
2NH3↑+ CaCl2+ 2H2O (2分) CO2↑+ 2SO2↑+ 2H2O (2分)
CO2↑+ 2SO2↑+ 2H2O (2分)